Effect of Acute Caffeine Intake on the Fat Oxidation Rate during Exercise: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection of Studies
2.2. Data Extraction
2.3. Quality Assessment of the Experiments
2.4. Statistical Analyses
3. Results
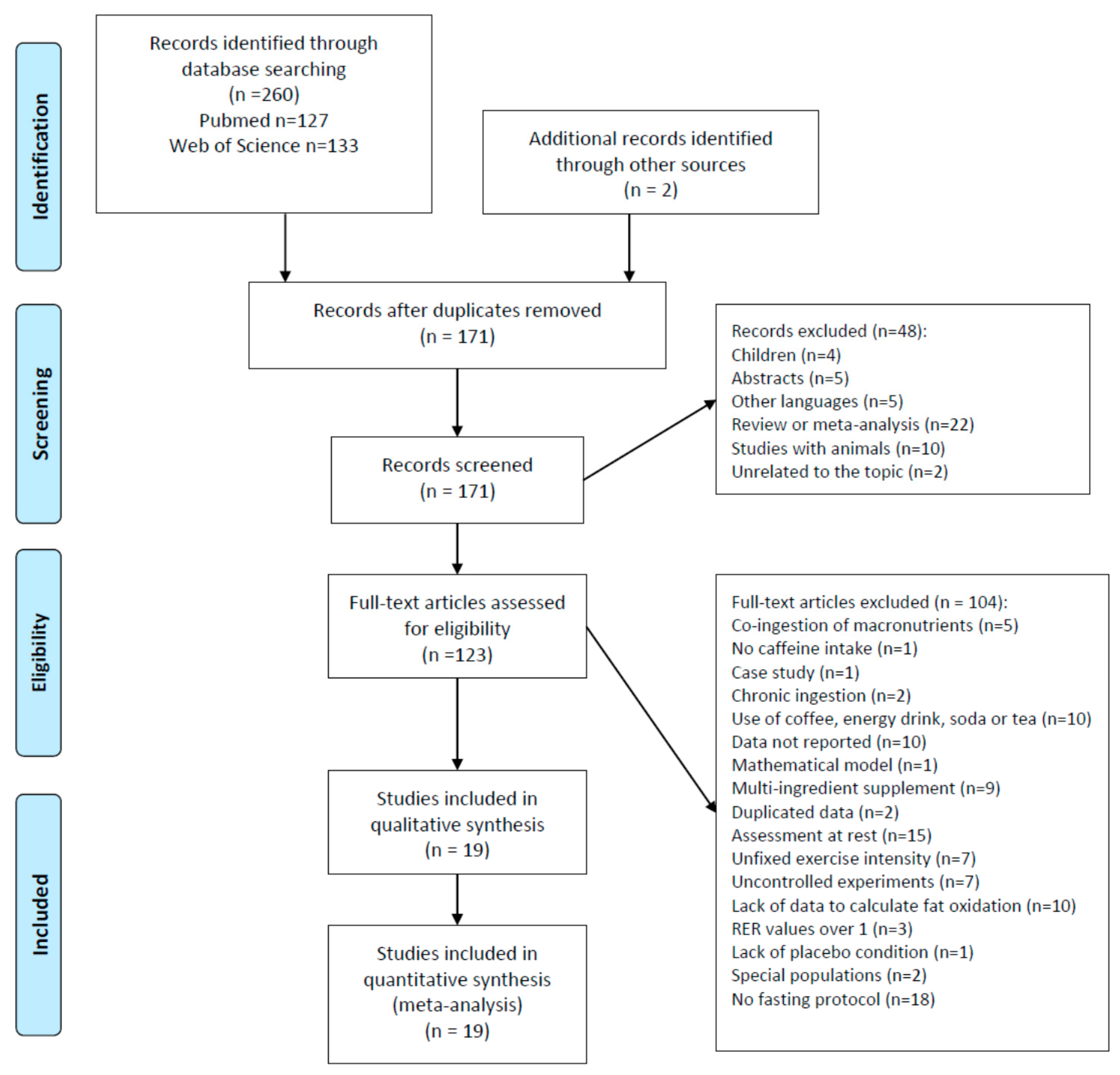
3.1. Study Selection
3.2. Risk of Bias
3.3. Descriptions of the Participants’ Characteristics
3.4. Descriptions of the Intervention Characteristics
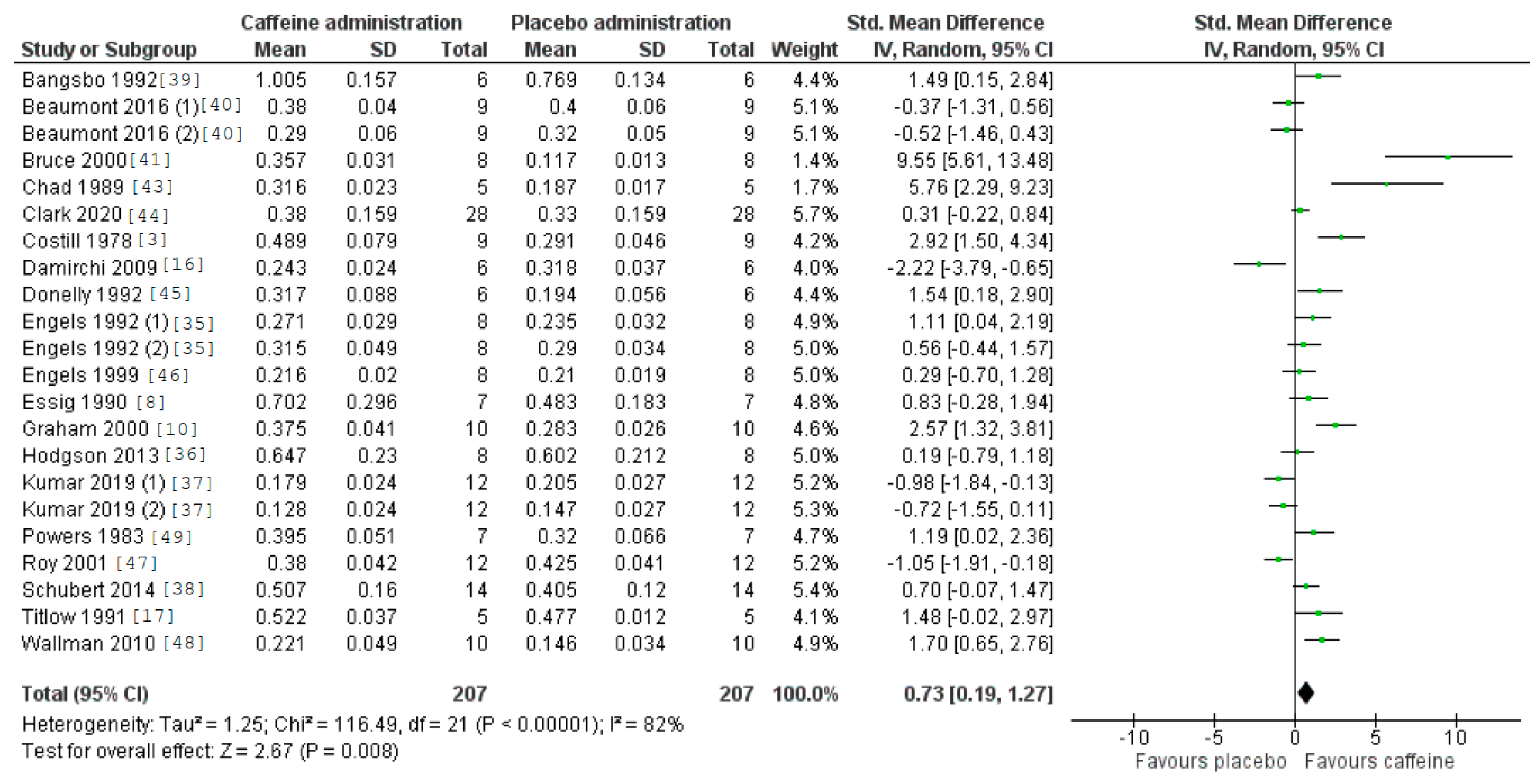
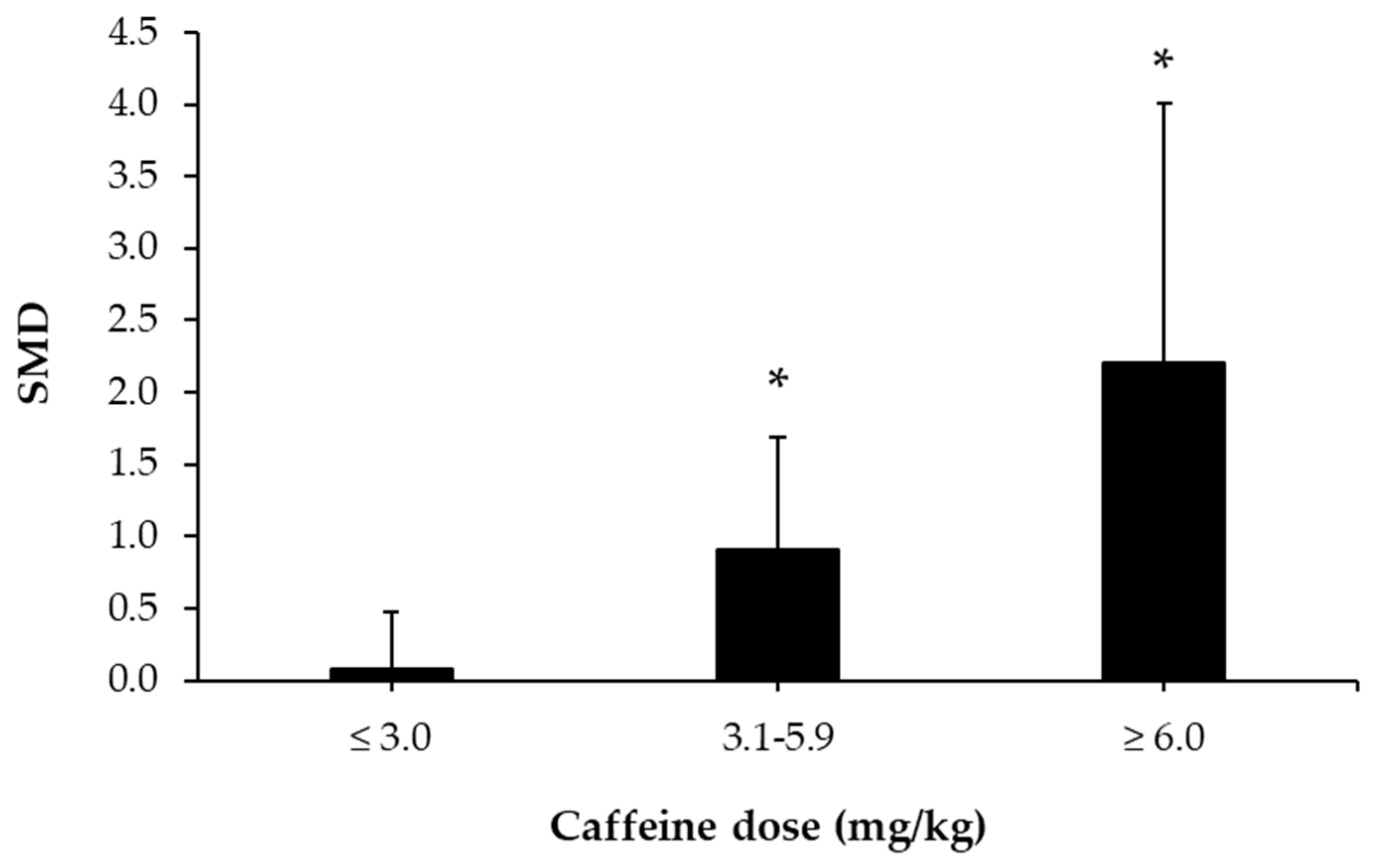
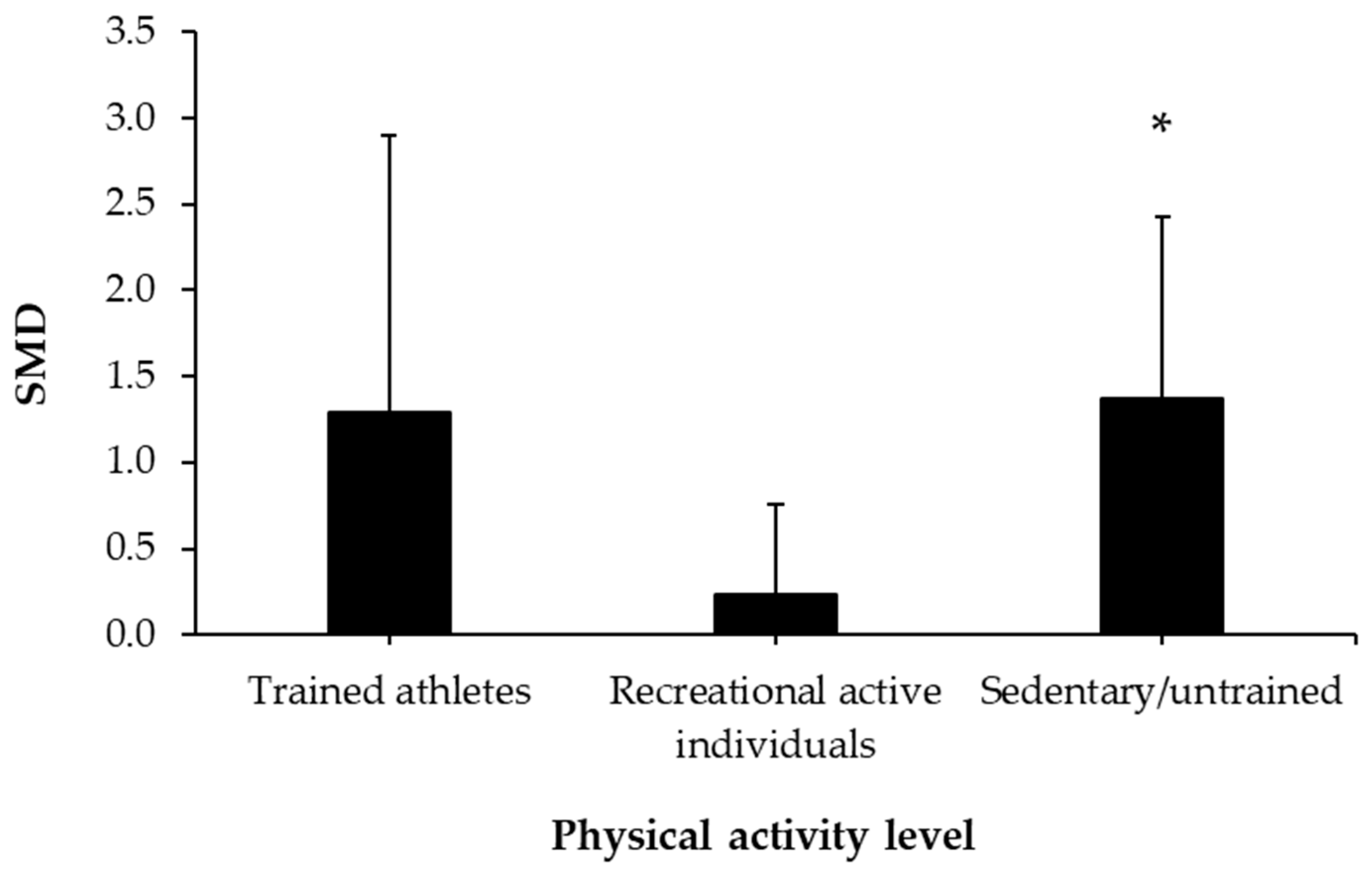
3.5. Caffeine Effects during Exercise
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Study | Intake | Form | Dose | Protocol | Device | Testing | Fasting |
|---|---|---|---|---|---|---|---|
| Bangsbo 1992 [39] | 30 min before exercise | 5 × 100 mg caffeine tablets | 500 mg (~7mg/kg) | 40 min at 80% VO2max | TRE | CONT | Overnight |
| Beaumont 2016 [40] | 60 min before exercise | Capsule | 3 mg/kg | 60 min at 60% VO2peak | CYC | CONT | Overnight |
| Bruce 2000 [41] | ~40–50 min before exercise | Capsule | 6 mg/kg | 6 min at 80% HRpeak | ROW | CONT | 12 h fast |
| Chad 1989 [43] | 60 min before exercise | Caffeine-containing juice | 5 mg/kg | 90 min of walking at 55% VO2max | TRE | CONT | >5 h |
| Clark 2020 [44] | ~90 min before exercise | Caffeine-containing drink | 140 mg (~2 mg/kg) | GXT (M: 35 W/3min and F: 25 W/3 min) | CYC | INC | 8 h |
| Costill 1978 [3] | 60 min before exercise | Decaffeinated coffee plus caffeine | 330 mg (~4.7 mg/kg) | 80% VO2max until exhaustion | CYC | CONT | 6–12 h |
| Damirchi 2009 [16] | 60 min before exercise | Capsule | 5 mg/kg | 30 min at 60% VO2max | TRE | CONT | Overnight |
| Donelly 1992 [45] | 60 min before exercise | Caffeine-containing drink | 5 mg/kg | 90 min at 55% VO2max | CYC | CONT | Overnight |
| Engels 1992 [35] | 60 min before exercise | Caffeine-containing drink | 5 mg/kg | 60 min walking at 30% and at 50% VO2max | TRE | CONT | Overnight |
| Engels 1999 [46] | 60 min before exercise | Caffeine-free diet cola plus caffeine | 5 mg/kg | 60 min at 30% VO2max | CYC | CONT | Overnight |
| Essig 1980 [8] | 60 min before exercise | Caffeine-containing drink | 5 mg/kg | 30 min at ~70% VO2max | CYC | CONT | 12 h |
| Graham 2000 [10] | 60 min before exercise | Capsule | 6 mg/kg | 60 min at 70% VO2max. | CYC | CONT | Overnight |
| Hodgson 2013 [36] | 60 min before exercise | Decaffeinated coffee plus caffeine | 5 mg/kg | 30 min at 50% Wmax (~55% VO2max). | CYC | CONT | Overnight |
| Kumar 2019 [37] | 40 min before exercise | Caffeine-containing drink | 3 mg/kg | 25 min at 10% below LT | CYC | CONT | Overnight |
| Powers 1983 [49] | 60 min before exercise | Capsule | 5 mg/kg | GXT with 30 W/3 min | CYC | INC | 6 h |
| Roy 2001 [47] | 75 min before exercise | Capsule | 6 mg/kg | 60 min at 65% VO2peak | CYC | CONT | 10 h |
| Schubert 2014 [38] | 60 min before exercise | Caffeine-containing drink | 3 mg/kg | 60 min at 60% VO2max | CYC | CONT | Overnight |
| Titlow 1991 [17] | 60 min before exercise | Capsules | 200 mg (~2.4 mg/kg) | 60 min at 60% HRmax | TRE | CONT | Overnight |
| Wallman 2010 [48] | 60 min before exercise | Capsule | 6 mg/kg | 15 min at 65% HRmax | CYC | CONT | 12 h fast |
References
- Reyes, C.M.; Cornelis, M.C. Caffeine in the Diet: Country-Level Consumption and Guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Navarro, M.; Muñoz, G.; Salinero, J.J. Urine Caffeine Concentration in Doping Control Samples from 2004 to 2015. Nutrients 2019, 11, 286. [Google Scholar] [CrossRef] [Green Version]
- Costill, D.L.; Dalsky, G.P.; Fink, W.J. Effects of caffeine ingestion on metabolism and exercise performance. Med. Sci. Sports 1978, 10, 155–158. [Google Scholar] [PubMed]
- Grgic, J. Caffeine ingestion enhances Wingate performance: A meta-analysis. Eur. J. Sport Sci. 2018, 18, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Trexler, E.T.; Lazinica, B.; Pedisic, Z. Effects of caffeine intake on muscle strength and power: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinero, J.J.; Lara, B.; Del Coso, J. Effects of acute ingestion of caffeine on team sports performance: A systematic review and meta-analysis. Res. Sports Med. 2019, 27, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Lara, B.; Ruiz-Vicente, D.; Areces, F.; Abián-Vicén, J.; Salinero, J.J.; Gonzalez-Millán, C.; Gallo-Salazar, C.; Del Coso, J. Acute consumption of a caffeinated energy drink enhances aspects of performance in sprint swimmers. Br. J. Nutr. 2015, 114, 908–914. [Google Scholar] [CrossRef] [Green Version]
- Essig, D.; Costill, D.; Van Handel, P. Effects of caffeine ingestion on utilization of muscle glycogen and lipid during leg ergometer cycling. Int. J. Sports Med. 1980, 1, 86–90. [Google Scholar] [CrossRef]
- Ivy, J.L.; Costill, D.L.; Fink, W.J.; Lower, R.W. Influence of caffeine and carbohydrate feedings on endurance performance. Med. Sci. Sports 1979, 11, 6–11. [Google Scholar] [CrossRef]
- Graham, T.E.; Helge, J.W.; MacLean, D.A.; Kiens, B.; Richter, E.A. Caffeine ingestion does not alter carbohydrate or fat metabolism in human skeletal muscle during exercise. J. Physiol. 2000, 529, 837. [Google Scholar] [CrossRef]
- Greer, F.; Friars, D.; Graham, T.E. Comparison of caffeine and theophylline ingestion: Exercise metabolism and endurance. J. Appl. Physiol. 2000, 89, 1837–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.M.; Zhao, Z.; Stock, H.S.; Mehl, K.A.; Buggy, J.; Hand, G.A. Central nervous system effects of caffeine and adenosine on fatigue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R399–R404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmenhorst, D.; Meyer, P.T.; Matusch, A.; Winz, O.H.; Bauer, A. Caffeine occupancy of human cerebral A1 adenosine receptors: In vivo quantification with 18F-CPFPX and PET. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2012, 53, 1723–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaister, M.; Pattison, J.R.; Muniz-Pumares, D.; Patterson, S.D.; Foley, P. Effects of dietary nitrate, caffeine, and their combination on 20-km cycling time trial performance. J. Strength Cond. Res. 2015, 29, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Green, J.M.; Olenick, A.; Eastep, C.; Winchester, L. Caffeine effects on velocity selection and physiological responses during RPE production. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Et Metab. 2016, 41, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Damirchi, A.; Rahmani-Nia, F.; Mirzaie, B.; Hasan-Nia, S.; Ebrahimi, M. Effect of caffeine on metabolic and cardiovascular responses to submaximal exercise in lean and obese men. Biomed. Hum. Kinet. 2009, 1, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Titlow, L.W.; Ishee, J.H.; Riggs, C.E. Failure of caffeine to affect metabolism during 60 min submaximal exercise. J. Sports Sci. 1991, 9, 15–22. [Google Scholar] [CrossRef]
- Gutiérrez-Hellín, J.; Del Coso, J. Effects of p-Synephrine and Caffeine Ingestion on Substrate Oxidation during Exercise. Med. Sci. Sports Exerc. 2018, 50, 1899–1906. [Google Scholar] [CrossRef]
- Cruz, R.S.; de Aguiar, R.A.; Turnes, T.; Guglielmo, L.G.; Beneke, R.; Caputo, F. Caffeine Affects Time to Exhaustion and Substrate Oxidation during Cycling at Maximal Lactate Steady State. Nutrients 2015, 7, 5254–5264. [Google Scholar] [CrossRef] [Green Version]
- Oskarsson, J.; McGawley, K. No individual or combined effects of caffeine and beetroot-juice supplementation during submaximal or maximal running. Appl. Physiol. Nutr. Metab. 2018, 43, 697–703. [Google Scholar] [CrossRef]
- Alkhatib, A.; Seijo, M.; Larumbe, E.; Naclerio, F. Acute effectiveness of a “fat-loss” product on substrate utilization, perception of hunger, mood state and rate of perceived exertion at rest and during exercise. J. Int. Soc. Sports Nutr. 2015, 12, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, J.R.; Camic, C.L.; Jagim, A.R. Effects of One Versus Two Doses of a Multi-Ingredient Pre-Workout Supplement on Metabolic Factors and Perceived Exertion during Moderate-Intensity Running in Females. Sports 2020, 8, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, T.E.; Spriet, L.L. Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. J. Appl. Physiol. 1995, 78, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.L.; Febbraio, M.A.; Arkinstall, M.J.; Hawley, J.A. Effect of caffeine co-ingested with carbohydrate or fat on metabolism and performance in endurance-trained men. Exp. Physiol. 2001, 86, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Yeo, S.E.; Jentjens, R.L.; Wallis, G.A.; Jeukendrup, A.E. Caffeine increases exogenous carbohydrate oxidation during exercise. J. Appl. Physiol. 2005, 99, 844–850. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Rohatgi, A. WebPlotdigitizer. 2018. Available online: https://automeris.io/WebPlotDigitizer (accessed on 15 October 2020).
- Brouwer, E. On simple formulae for calculating the heat expenditure and the quantities of carbohydrate and fat oxidized in metabolism of men and animals, from gaseous exchange (Oxygen intake and carbonic acid output) and urine-N. Acta Physiol. et Pharmacol. Neerl. 1957, 6, 795–802. [Google Scholar]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Baltazar-Martins, J.G.; De Souza, D.B.; Aguilar, M.; Grgic, J.; Del Coso, J. Infographic. The road to the ergogenic effect of caffeine on exercise performance. Br. J. Sports Med. 2020, 54, 618–619. [Google Scholar] [CrossRef]
- Spriet, L.L. Exercise and sport performance with low doses of caffeine. Sports Med. 2014, 44, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, F.L.; Oh, I.S.; Hayes, T.L. Fixed-versus random-effects models in meta-analysis: Model properties and an empirical comparison of differences in results. Br. J. Math. Stat. Psychol. 2009, 62, 97–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, D.L.; Guyatt, G.H.; Acquadro, C. Patient-reported outcomes. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; The Cochrane Collaboration: London, UK, 2008; pp. 531–545. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Engels, H.J.; Haymes, E.M. Effects of caffeine ingestion on metabolic responses to prolonged walking in sedentary males. Int. J. Sport Nutr. 1992, 2, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.B.; Randell, R.K.; Jeukendrup, A.E. The metabolic and performance effects of caffeine compared to coffee during endurance exercise. PLoS ONE 2013, 8, e59561. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Warren, G.L.; Snow, T.K.; Millard-Stafford, M. Caffeine ingestion with or without low-dose carbohydrate improves exercise tolerance in sedentary adults. Front. Nutr. 2019, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.M.; Hall, S.; Leveritt, M.; Grant, G.; Sabapathy, S.; Desbrow, B. Caffeine consumption around an exercise bout: Effects on energy expenditure, energy intake, and exercise enjoyment. J. Appl. Physiol. 2014, 117, 745–754. [Google Scholar] [CrossRef]
- Bangsbo, J.; Jacobsen, K.; Nordberg, N.; Christensen, N.J.; Graham, T. Acute and habitual caffeine ingestion and metabolic responses to steady-state exercise. J. Appl. Physiol. 1992, 72, 1297–1303. [Google Scholar] [CrossRef]
- Beaumont, R.; Cordery, P.; Funnell, M.; Mears, S.; James, L.; Watson, P. Chronic ingestion of a low dose of caffeine induces tolerance to the performance benefits of caffeine. J. Sports Sci. 2017, 35, 1920–1927. [Google Scholar] [CrossRef]
- Bruce, C.R.; Anderson, M.E.; Fraser, S.F.; Stepto, N.K.; Klein, R.; Hopkins, W.G.; Hawley, J.A. Enhancement of 2000-m rowing performance after caffeine ingestion. Med. Sci. Sports Exerc. 2000, 32, 1958–1963. [Google Scholar] [CrossRef] [Green Version]
- Casal, D.C.; Leon, A.S. Failure of caffeine to affect substrate utilization during prolonged running. Med. Sci. Sports Exerc. 1985, 17, 174–179. [Google Scholar] [CrossRef]
- Chad, K.; Quigley, B. The effects of substrate utilization, manipulated by caffeine, on post-exercise oxygen consumption in untrained female subjects. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 59, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.W.; Wells, A.J.; Coker, N.A.; Goldstein, E.R.; Herring, C.H.; Starling-Smith, T.M.; Varanoske, A.N.; Panissa, V.L.G.; Stout, J.R.; Fukuda, D.H. The acute effects of thermogenic fitness drink formulas containing 140 mg and 100 mg of caffeine on energy expenditure and fat metabolism at rest and during exercise. J. Int. Soc. Sports Nutr. 2020, 17, 10. [Google Scholar] [CrossRef] [Green Version]
- Donelly, K.; McNaughton, L. The effects of two levels of caffeine ingestion on excess postexercise oxygen consumption in untrained women. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Engels, H.-J.; Wirth, J.C.; Celik, S.; Dorsey, J.L. Influence of caffeine on metabolic and cardiovascular functions during sustained light intensity cycling and at rest. Int. J. Sport Nutr. Exerc. Metab. 1999, 9, 361–370. [Google Scholar] [CrossRef]
- Roy, B.; Bosman, M.; Tarnopolsky, M. An acute oral dose of caffeine does not alter glucose kinetics during prolonged dynamic exercise in trained endurance athletes. Eur. J. Appl. Physiol. 2001, 85, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Wallman, K.E.; Goh, J.W.; Guelfi, K.J. Effects of caffeine on exercise performance in sedentary females. J. Sports Sci. Med. 2010, 9, 183–189. [Google Scholar] [PubMed]
- Powers, S.; Byrd, R.; Tulley, R.; Callender, T. Effects of caffeine ingestion on metabolism and performance during graded exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 50, 301–307. [Google Scholar] [CrossRef]
- Toner, M.; Kirkendall, D.; Delio, D.; Chase, J.; Cleary, P.; Fox, E. Metabolic and cardiovascular responses to exercise with caffeine. Ergonomics 1982, 25, 1175–1183. [Google Scholar] [CrossRef]
- Graham, T.E.; Battram, D.S.; Dela, F.; El-Sohemy, A.; Thong, F.S. Does caffeine alter muscle carbohydrate and fat metabolism during exercise? Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Et Metab. 2008, 33, 1311–1318. [Google Scholar] [CrossRef]
- Spriet, L.L.; MacLean, D.A.; Dyck, D.J.; Hultman, E.; Cederblad, G.; Graham, T.E. Caffeine ingestion and muscle metabolism during prolonged exercise in humans. Am. J. Physiol. 1992, 262, E891–E898. [Google Scholar] [CrossRef]
- Ruiz-Moreno, C.; Gutiérrez-Hellín, J.; Amaro-Gahete, F.J.; González-García, J.; Giráldez-Costas, V.; Pérez-García, V.; Del Coso, J. Caffeine increases whole-body fat oxidation during 1 h of cycling at Fatmax. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E. Caffeine, coffee and ephedrine: Impact on exercise performance and metabolism. Can. J. Appl. Physiol. Rev. Can. Physiol. Appl. 2001, 26, S103–S119. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Şahin, M.A.; Cook, M.D. Matcha Green Tea Drinks Enhance Fat Oxidation during Brisk Walking in Females. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glaister, M.; Gissane, C. Caffeine and Physiological Responses to Submaximal Exercise: A Meta-Analysis. Int. J. Sports Physiol. Perform. 2018, 13, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Kavouras, S.A. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2005, 45, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Sadek, P.; Pan, X.; Shepherd, P.; Malandain, E.; Carney, J.; Coleman, H. A Randomized, Two-Way Crossover Study to Evaluate the Pharmacokinetics of Caffeine Delivered Using Caffeinated Chewing Gum Versus a Marketed Caffeinated Beverage in Healthy Adult Volunteers. J. Caffeine. Res. 2017, 7, 125–132. [Google Scholar] [CrossRef]
- Salinero, J.J.; Lara, B.; Abian-Vicen, J.; Gonzalez-Millán, C.; Areces, F.; Gallo-Salazar, C.; Ruiz-Vicente, D.; Del Coso, J. The use of energy drinks in sport: Perceived ergogenicity and side effects in male and female athletes. Br. J. Nutr. 2014, 112, 1494–1502. [Google Scholar] [CrossRef] [Green Version]
- Pasman, W.J.; Van Baak, M.A.; Jeukendrup, A.E.; De Haan, A. The effect of different dosages of caffeine on endurance performance time. Int. J. Sports Med. 1995, 16, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Filip, A.; Wilk, M. Inconsistency in the Ergogenic Effect of Caffeine in Athletes Who Regularly Consume Caffeine: Is It Due to the Disparity in the Criteria That Defines Habitual Caffeine Intake? Nutrients 2020, 12, 1087. [Google Scholar] [CrossRef] [Green Version]
- Lara, B.; Ruiz-Moreno, C.; Salinero, J.J. Time course of tolerance to the performance benefits of caffeine. PLoS ONE 2019, 14, e0210275. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Moreno, C.; Lara, B.; Salinero, J.J.; Brito De Souza, D.; Ordovás, J.M.; Del Coso, J. Time course of tolerance to adverse effects associated with the ingestion of a moderate dose of caffeine. Eur. J. Nutr. 2020, 59, 3293–3302. [Google Scholar] [CrossRef] [PubMed]
- Collomp, K.; Ahmaidi, S.; Chatard, J.C.; Audran, M.; Préfaut, C. Benefits of caffeine ingestion on sprint performance in trained and untrained swimmers. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; Cottrell, T.; Talhami Lozano, A.; Aburto-Pratt, K.; Duhon, J. Effect of caffeine on RPE and perceptions of pain, arousal, and pleasure/displeasure during a cycling time trial in endurance trained and active men. Physiol. Behav. 2012, 106, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.H.; Wyld, K.; Chrismas, B.C. Acute effects of caffeine on strength performance in trained and untrained individuals. J. Athl. Enhanc. 2015, 4. [Google Scholar] [CrossRef]
- Skinner, T.L.; Jenkins, D.G.; Leveritt, M.D.; McGorm, A.; Bolam, K.A.; Coombes, J.S.; Taaffe, D.R. Factors influencing serum caffeine concentrations following caffeine ingestion. J. Sci. Med. Sport 2014, 17, 516–520. [Google Scholar] [CrossRef]
- Doherty, M.; Smith, P.M.; Davison, R.C.; Hughes, M.G. Caffeine is ergogenic after supplementation of oral creatine monohydrate. Med. Sci. Sports Exerc. 2002, 34, 1785–1792. [Google Scholar] [CrossRef]
- Grgic, J.; Mikulic, P. Caffeine ingestion acutely enhances muscular strength and power but not muscular endurance in resistance-trained men. Eur. J. Sport Sci. 2017, 17, 1029–1036. [Google Scholar] [CrossRef]
- Pickering, C.; Kiely, J. Are the Current Guidelines on Caffeine Use in Sport Optimal for Everyone? Inter-individual Variation in Caffeine Ergogenicity, and a Move Towards Personalised Sports Nutrition. Sports Med. 2018, 48, 7–16. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Marques-Jiménez, D.; Refoyo, I.; Del Coso, J. Effect of Caffeine Supplementation on Sports Performance Based on Differences between Sexes: A Systematic Review. Nutrients 2019, 11, 2313. [Google Scholar] [CrossRef] [Green Version]
- Skinner, T.L.; Desbrow, B.; Arapova, J.; Schaumberg, M.A.; Osborne, J.; Grant, G.D.; Anoopkumar-Dukie, S.; Leveritt, M.D. Women Experience the Same Ergogenic Response to Caffeine as Men. Med. Sci. Sports Exerc. 2019, 51, 1195–1202. [Google Scholar] [CrossRef] [Green Version]
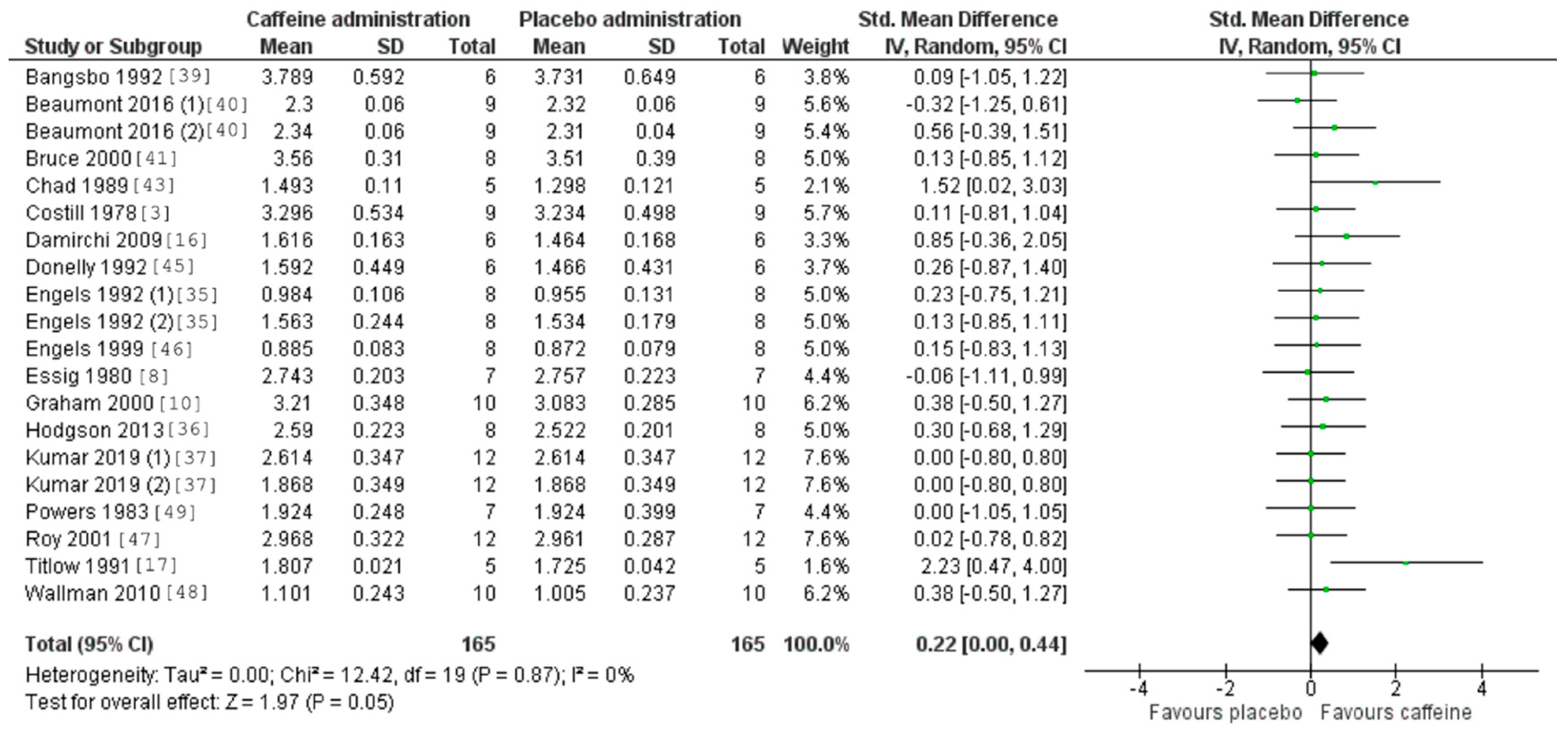
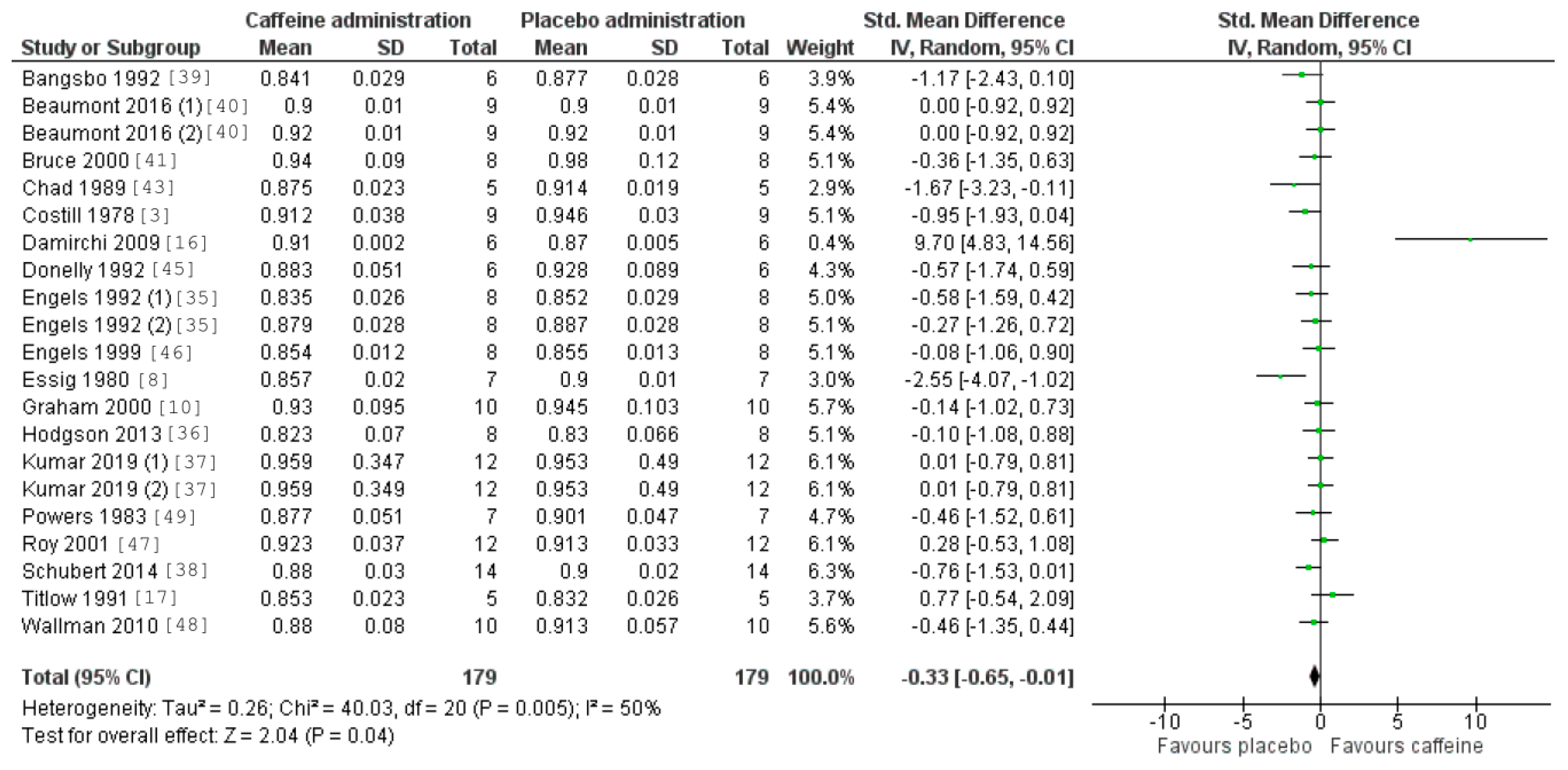
| Study/Item | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bangsbo 1992 [39] | N | Y | N | Y | N | N | N | Y | Y | Y | Y | 6/10 |
| Beaumont 2016 [40] | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10/10 |
| Bruce 2000 [41] | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 9/10 |
| Chad 1989 [43] | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 9/10 |
| Clark 2020 [44] | N | Y | Y | Y | Y | Y | N | Y | N | Y | Y | 8/10 |
| Costill 1978 [3] | N | Y | N | Y | Y | N | N | Y | Y | Y | Y | 8/10 |
| Damirchi 2009 [16] | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 9/10 |
| Donelly 1992 [45] | N | N | Y | Y | Y | Y | N | Y | Y | Y | Y | 8/10 |
| Engels 1992 [35] | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 9/10 |
| Engels 1999 [46] | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 9/10 |
| Essig 1980 [8] | N | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 8/10 |
| Graham 2000 [10] | N | N | Y | Y | Y | Y | N | Y | Y | Y | Y | 8/10 |
| Hodgson 2013 [36] | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 9/10 |
| Kumar 2019 [37] | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y | 8/10 |
| Powers 1983 [49] | N | N | Y | Y | Y | N | N | Y | Y | Y | Y | 7/10 |
| Roy 2001 [47] | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 9/10 |
| Schubert 2014 [38] | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 9/10 |
| Titlow 1991 [17] | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 9/10 |
| Wallman 2010 [48] | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 9/10 |
| Study | Design | Age (Years) | Sample Size | Physical Activity Level | Habitual Caffeine Intake |
|---|---|---|---|---|---|
| Bangsbo 1992 [39] | RDB | 33 (26–39) | 6 | Long-distance runners with 6 years of experience. Training volume ~58 km/week | 6 (0–8) cups of coffee |
| Beaumont 2016 [40] | RDB | 21 ± 2 | 18 men | Recreationally active practitioners | <75 mg/day |
| Bruce 2000 [41] | RDB | Not reported | 8 men | Well and regularly trained rowers | Not reported |
| Chad 1989 [43] | RDB | 21 ± 1.5 | 5 women | Untrained (sedentary) individuals | Unhabituated |
| Clark 2020 [44] | RDB | 22.9 ± 0.7 (18–35) | 32 men (15) and women (17) | Recreationally active individuals, with less than 150 min/week of exercise in the last 6 months | 832 ± 69 mg/week |
| Costill 1978 [3] | RSB | Men: 22.4; Women: 20.5 | 9 men (7) and women (2) | Competitive cyclists | Not reported |
| Damirchi 2009 [16] | RDB | 21.8 ± 1.3 | 6 men | Participants in leisure physical activities (not competitive) | >300 mg/day |
| Donelly 1992 [45] | RDB | 20.5 (SEM 0.5) | 6 women | Untrained participants | <220 mg/day |
| Engels 1992 [35] | RDB | 23.5 (21–28) | 8 men | Sedentary individuals with VO2max between 30 and 45 mL/kg/min | 125 mg/day |
| Engels 1999 [46] | RDB | 26.9 (SEM 1.4) | 8 adults (1 woman) | Recreational practitioners (not competitive athletes) | 240.9 mg (SEM 57.8) mg/day |
| Essig 1980 [8] | RSB | 24.7 ± 2.2 | 7 men | Active individuals | Not reported |
| Graham 2000 [10] | DB | 25.7 (20–28) | 10 men | Healthy individuals | Not reported |
| Hodgson 2013 [36] | RSB | 41 ± 7 | 8 men | Cyclists or triathletes who trained 3 or more times per week (90–min/session) in the previous two years | ≤300 mg/day |
| Kumar 2019 [37] | DB | Athletes group: 27.7 ± 5.5; sedentary group: 26.8 ± 7.0 | 12 athletes and 12 healthy sedentary men (10) and women (2) | Endurance cycling, triathlon, and cross-country athletes practicing >360 min/week of exercise training | <500 mg/day |
| Powers 1983 [49] | SB | 28 (SEM 1.1) | 7 men | Recreationally trained bicyclists with 4 to 7 sessions/week. | Not reported |
| Roy 2001 [47] | RDB | Not reported | 12, 7 men and 5 women | Endurance athletes with VO2max > 50 and 60 mL/ki/min for women and men, respectively. | Not reported |
| Schubert 2014 [38] | RDB | 24.9 ± 4.4 | 14 women (8) and men (6) | Recreational exercise practitioners of ≥30 min of moderate-intensity exercise/day, ≥3 days/week | 206 ± 194 mg/day |
| Titlow 1991 [17] | RDB | 25.0 ± 3.6 | 5 men | Involved in programs of running, cycling, and a variety of sports and games. | Not reported |
| Wallman 2010 [48] | RDB | 22 ± 2 | 10 women | Healthy sedentary practicing < 20 min of exercise, <3 days/week | <100 mg/day |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collado-Mateo, D.; Lavín-Pérez, A.M.; Merellano-Navarro, E.; Coso, J.D. Effect of Acute Caffeine Intake on the Fat Oxidation Rate during Exercise: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 3603. https://doi.org/10.3390/nu12123603
Collado-Mateo D, Lavín-Pérez AM, Merellano-Navarro E, Coso JD. Effect of Acute Caffeine Intake on the Fat Oxidation Rate during Exercise: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(12):3603. https://doi.org/10.3390/nu12123603
Chicago/Turabian StyleCollado-Mateo, Daniel, Ana Myriam Lavín-Pérez, Eugenio Merellano-Navarro, and Juan Del Coso. 2020. "Effect of Acute Caffeine Intake on the Fat Oxidation Rate during Exercise: A Systematic Review and Meta-Analysis" Nutrients 12, no. 12: 3603. https://doi.org/10.3390/nu12123603






